Description
Leading innovation in lipid layer thickness measurement and meibomian gland imaging.
Benefits:
-
Real-time visualization of the lipid layer to evaluate the dynamic response of lipids to blinking
-
Patented noise canceling technology to measure sub-micron thickness of the lipid layer
-
Video and analysis of blink dynamics
-
High-definition imaging with Dynamic Meibomian Imaging
-
Room lights must be dimmed during testing
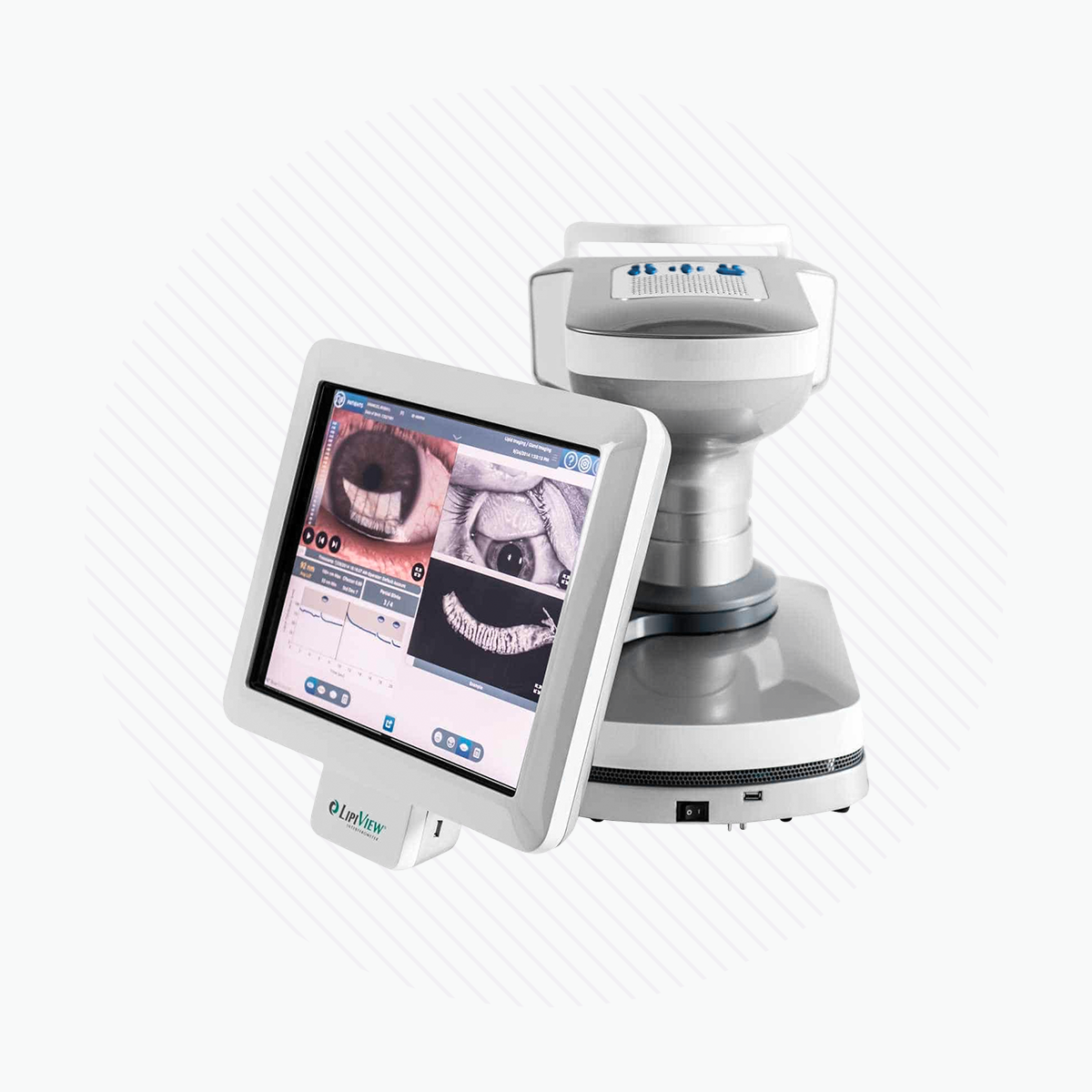
LipiView® II Ocular Surface Interferometer with Dynamic Meibomian Imaging™ (DMI) measures lipid layer thickness (LLT) with nanometer accuracy, captures blink dynamics, and images meibomian gland structure.
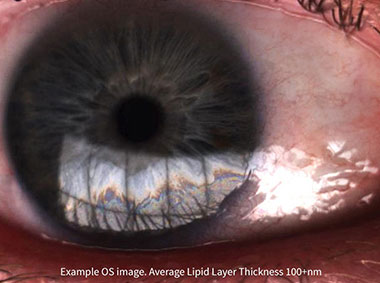
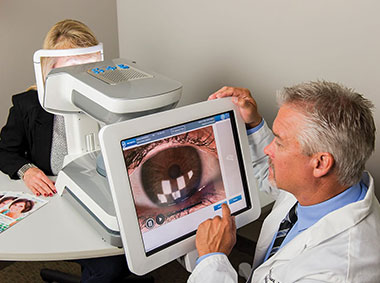
The LipiView® II Interferometer features patented technology that provides a sophisticated assessment of factors that contribute to dry eye. Compelling visuals and video captures provide a unique opportunity to educate patients about their personal ocular health.
Dynamic Meibomian Imaging
MEIBOMIAN GLANDS IN HIGH DEFINITION
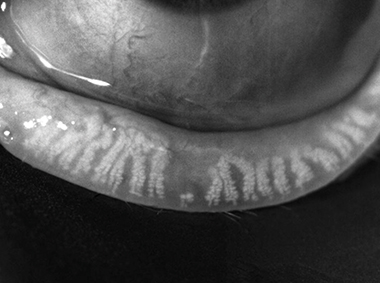
Dynamic Illumination
Surface lighting originates from multiple light sources to minimize reflection.
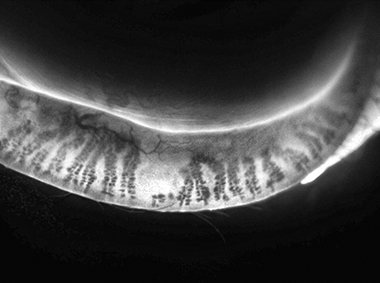
Adaptive Transillumination
Changes to the light intensity across the surface of the illuminator compensate for the lid thickness variations between patients.
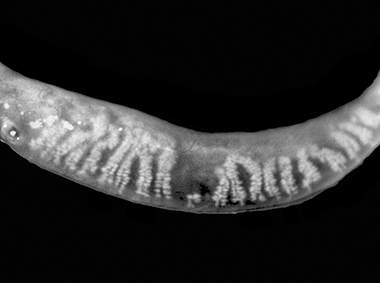
Dual-Mode DMI
A combination of Dynamic Illumination and Adaptive Transillumination offers an enhanced view of meibomian gland structure.
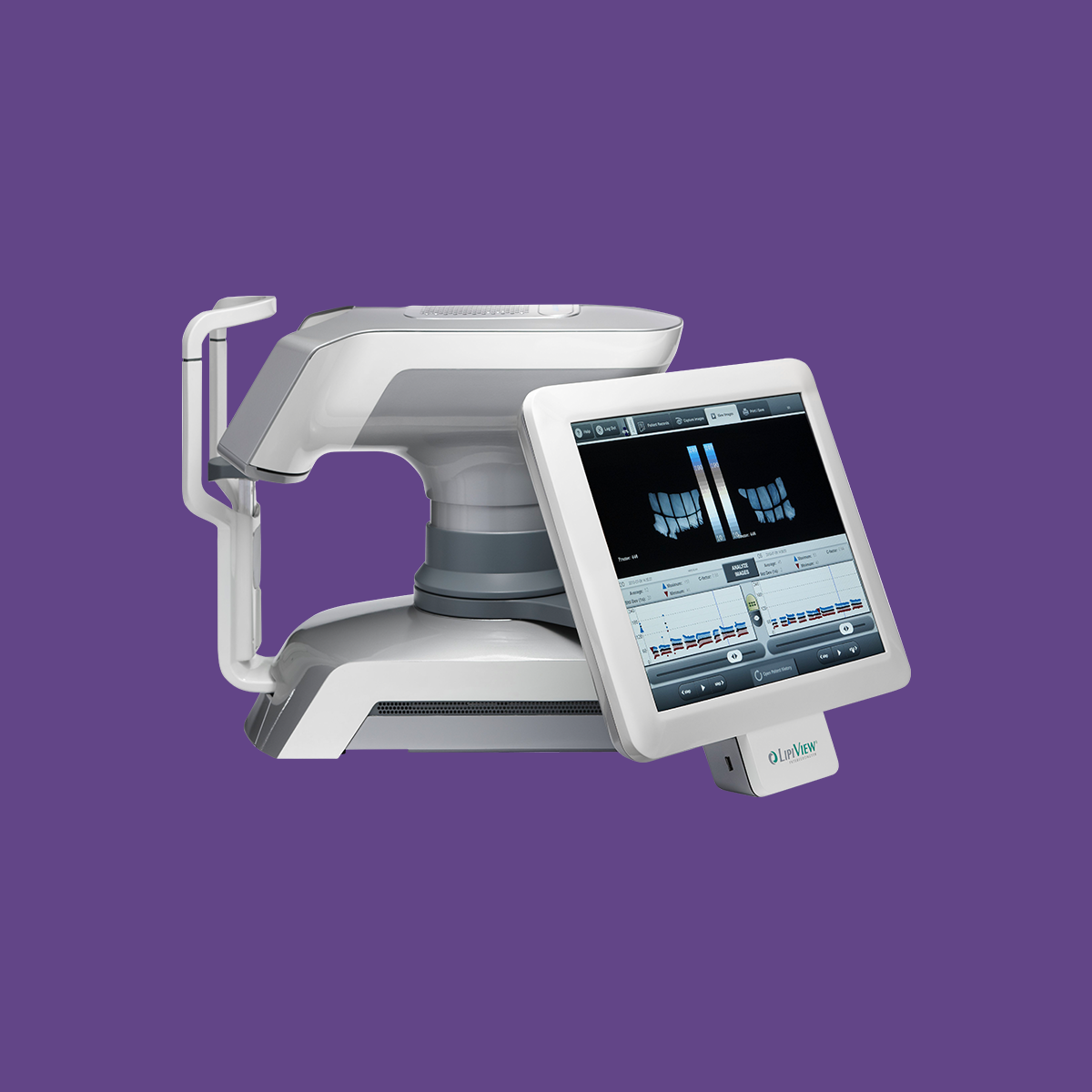
LipiView® II Reporting
REAL-TIME VISUALIZATION
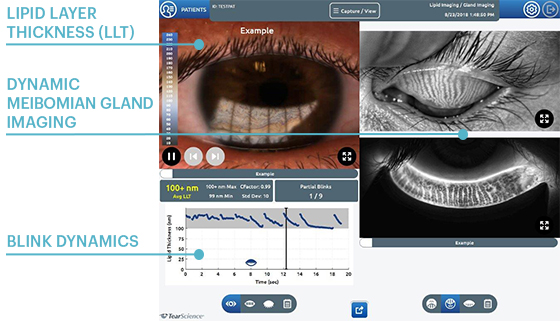
Lipid Layer Thickness (LLT)
Presents lipid layer thickness measurements in an easy to understand color-coded map.
Dynamic Meibomian Gland Imaging
Utilizes advanced illumination technology to capture high-definition images.
Blink Dynamics
Analyzes blink patterns and detects partial blinks. A graphical representation shows fluctuations in lipid layer thickness measurements between each blink.
Visualize and Analyze Blinks
Analyze Blink Patterns
Computerized analysis of blink patterns objectively quantifies the number of complete and partial blinks. Results are displayed in a frame-by-frame graphical representation that shows fluctuations in lipid thickness measurements between each blink. A video capture allows clinicians and patients to review blinking habits during consultation.
Normal vs Partial Blink
Visualize Blink Response
High-resolution video depicts how well ocular lipids disperse in response to a blink through variations in light patterns reflected off the tear film called an interferogram. The system enhances the interference pattern and displays a profile corresponding to an interferometry color scale which has been validated to a known standard for lipid layer thickness measurements with a precision of 1 nm and an accuracy of ±10 nm.
Lipid Imaging Mode
Benefits
LipiView® II Features
- Real-time visualization of the lipid layer to evaluate the dynamic response of lipids to blinking
- Patented noise canceling technology to measure sub-micron thickness of the lipid layer
- Video and analysis of blink dynamics
- High-definition imaging with Dynamic Meibomian Imaging
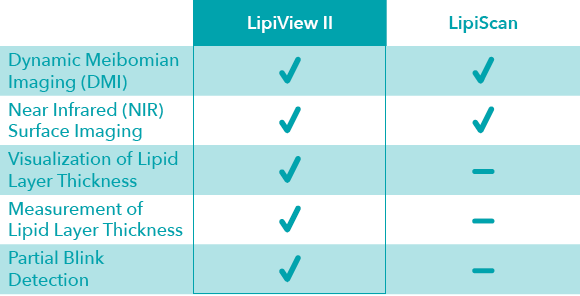
Feature Comparison
Indications & Safety Information
SUMMARY OF IMPORTANT SAFETY INFORMATION
Rx Only
FOR THE LIPIVIEW® II OCULAR SURFCE INTERFEROMETER
INDICATIONS FOR USE
The LipiView® II Ocular Surface Interferometer is an ophthalmic imaging device that is intended for use by a physician in adult patients to capture, archive, manipulate and store digital images of:
- Specular (interferometric) observations of the tear film. Using these images, LipiView® II measures the absolute thickness of the tear film lipid layer.
- Meibomian glands under near-infrared (NIR) illumination.
- The ocular surface and eyelids under white illumination.
CONTRAINDICATIONS
Contraindications are conditions in which the device should not be used because the risk of use clearly outweighs any benefit. No contraindications have been identified for LipiView® II.
PRECAUTIONS
The following patient conditions may affect the interferometry assessment of a patient’s tear film using LipiView® II:
- Use of ophthalmic drops such as artificial tear lubricants, ointments, and medications. Advise patients not not instill oil-based ophthalmic drops (e.g., Soothe®, Restasis®, Systane Balance®) for at least 12 hours prior to device use and not to instill ointments for at least 24 hours prior to device use. Wait at least four (4) hours after the instillation of all other ophthalmic drops prior to device use.
- Soft or rigid contact lens wear. Advise patients to remove contact lenses at least four hours prior to device use.
- Use of oil-based facial cosmetics around the eye.
- Eye rubbing.
- Recent swimming in a chlorinated pool. Advise pateints to not to swim for at least 12 hours prior to device use.
- Any ocular surface condition that affects the stability of the tear film. These conditions include disease, dystrophy, trauma, scarring, surgery, or abnormality.
POTENTIAL ADVERSE EFFECTS
There are no known or anticipated adverse effects associated with use of this device.