Description
AT A GLANCE
Treatment Points & Uses
- Re-epithelialization
- Cornel Abrasions
- Corneal Ulcers
- Severe Dry Eye
- Recurrent Corneal
- Erosion Filamentary Keratitis
- Chemical Burns
- Post Foreign Body Removal
- Superficial Keratectomy
- And Much More
Functional Characteristics
- Integrates into ocular tissue quickly
- Attaches well to ocular surface
- Easy for provider to put on patient
- Quick anti-inflammatory results
- Immune system quiet (no MHC II antigens)
Manufacturing Source Integrity
All Manufacturing of finished product performed in Tallahassee, Florida.
Placental amniotic tissue recovered by the largest tissue recovery company in the United States in over 50 hospitals.
ABOUT US
EyeDisc is a Florida company owned by eyecare industry executives and Eye Care Providers. Our Goal is to provide as many of our eye physician customers with a great dehydrated amniotic membrane that can be used to treat eye problems without substantial carrying costs.
Our partners at IntegoGen are a vertically integrated tissue biologics company that specializes in the recovery, processing and distribution of amniotic tissue allografts. This means that we control the quality of each tissue allograft throughout the entire continuum (tissue recovery, processing and distribution) as well as the costs associated.
PATIENT APPLICATION
Applying the EyeDisc to the eye is fairly simple and consists of two basic methods outlined below. Important note...the EyeDisc is orientation neutral in its application, meaning that either side of the membrane can be placed onto the corneal surface. EyeDisc will be publishing it's own set of videos and infographics for patient application in the near future. For now, these steps should be easy to follow.
APPLICATION OPTION A (For the Pragmatist):
- Place topical proparacaine on the ocular surface
- Open a bandage contact, take it out and place on sterile space
- Open the amniotic membrane pouch slowly until the amniotic membrane lens becomes exposed
- Using forceps, remove the lens from the pouch and place inside of the bandage contact lens
- Smooth the amniotic membrane disc into the contact lens
- Prepare the ocular surface of the patient as you normally would prior to a contact lens insertion
- Place the bandage contact lens onto the corneal surface
- Complete the enclosed Tissue Tracking Form and return it to our Tissue Bank
APPLICATION OPTION B (For the Purist):
- Recline patient and tilt chin up, then place topical proparacaine on the ocular surface
- Insert speculum to hold open eye and dry cornea
- Open the amniotic membrane pouch slowly until the amniotic membrane lens becomes exposed
- Using forceps, remove the amniotic membrane lens from the pouch and place over cornea
- Using forceps still, make sure the amniotic membrane lens is smoothly covering cornea and / or flat to cornea
- Place bandage contact onto the corneal surface
- Remove speculum
- Complete the enclosed Tissue Tracking Form and return it to our Tissue Bank
MATERIALS NEEDED
- Blunt Tip Sterile Forceps
- Proparacaine (or bio-similar topical anesthetic drop)
- Weck-Cel or Soft-Cel Spear Q-Tips
- Sterile gloves
- Sterile work space
- Bandage Contact Lens
WHAT IS AMNIOTIC MEMBRANE TISSUE?
Amniotic Membrane is human placental tissue product donated by birth
mothers giving birth through a planned Cesarean delivery. It is a thin tough sac of membrane that protects the embryo during pregnancy against any injuries
that can lead to fetal death. Used successfully for over 60 years as a biological bandaid, Amniotic Membrane tissue has become an emerging alternative for clinicians to rapidly and effectively treat a variety of ocular surface conditions. The Amniotic Membrane itself is avascular and contains cytokines and growth factors (epidermal, keratinocyte, transforming growth factor beta, vascular
endothelial growth factor and platelet derived growth factor). It is from these key growth factors and cytokines that Amniotic Membranes derive their
healing efficacy, but pursuant to safety, they also possess many anti-microbial and anti-inflammatory properties.
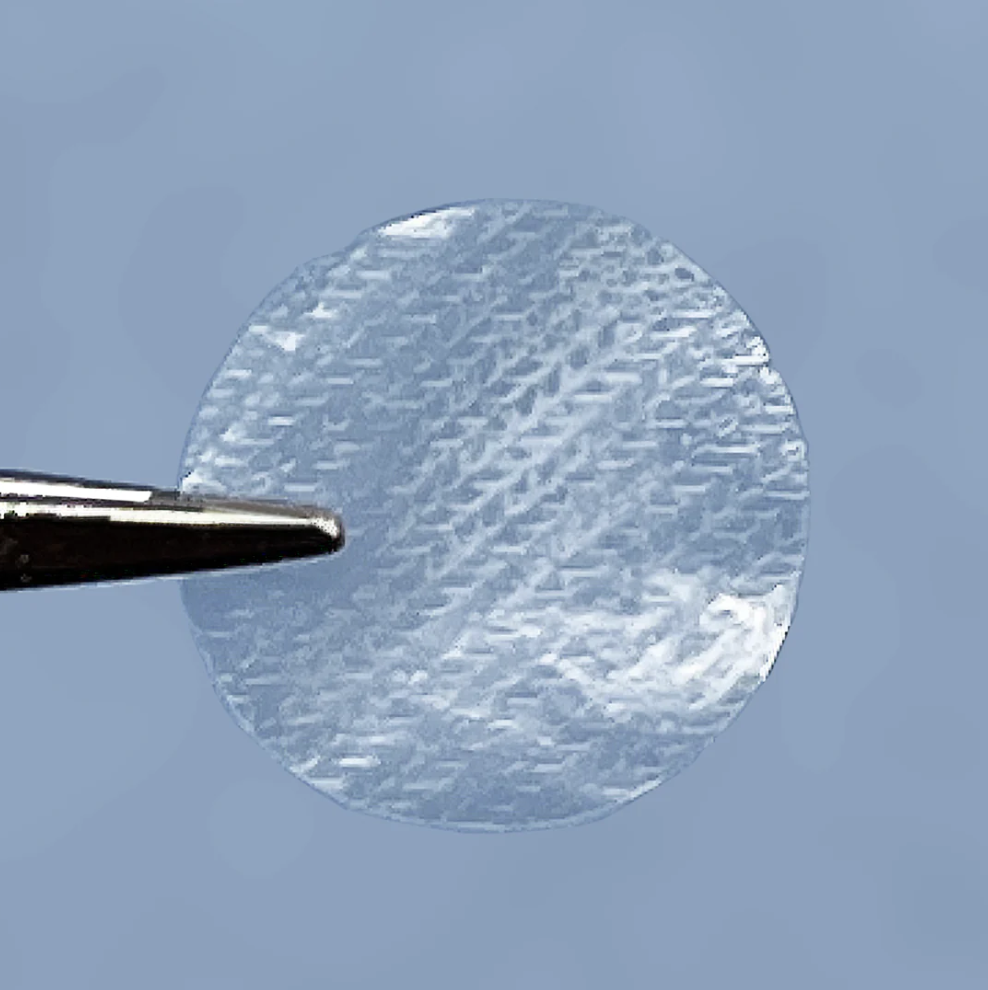
HOW ARE EYEDISCS MADE AND SIZED
During processing, EyeDiscs are simply cut out of a layer of amnion that has been spread across a sterile work table using a small tool. The end result is a group of uniform discs that are 8mm and 12mm in diameter. Larger sizes can be made available as the need arises.
STORAGE, DEHYDRATION AND PROCESSING
EyeDisc's Amniotic Membrane discs are easy to use, have a long shelf life, and achieve incredible results. They have been dehydrated for the purpose of shelf life via an Air Dried process. In addition to this drying process, EyeDisc(s) are
not subjected to harsh chemical sterilization, additives or preservatives, the
whole of which maintains cytokine and growth factor count, which are the key ingredients for quality and therapeutic integrity.
SCREENING & STERILITY
Tissue donations are screened for communicable disease by a laboratory
registered with the FDA to perform donor testing and certified to perform such testing on human specimens in accordance with Clinical Laboratory
Improvement Amendments (CLIA) and 42 CFR Part 493, or that has equivalent requirements as determined by the Centers for Medicare and Medicaid
Services. Donor tissue is recovered using the safest recovery techniques and sterile equipment to minimize any bioburden contamination and a highly
controlled regulatory and quality assurance processing environment, thus countering the risks of disease transmission at every step. Additionally,
through the use of "E-Beam" technology, which uses advanced electronics to precisely control the use of electrons in the sterilization process, unwanted microorganisms are eliminated.
The EyeDisc Amniotic Membrane Allografts are easy to use, absorb very well and are an overall high quality product that promote
healing and re-epithelialization...I like using them.
JoshDavidson, OD,FAAO
Williamson Eye Center
Price Match Guarantee
Best pricing for your practice, always.
Best pricing for your practice, always.