Description
Designed to Improve Gland Function
At-a-Glance
- Computer-controlled, simultaneous application of heat and pressure
- After an initial anesthetic drop, no drugs are required for the procedure
- The LipiFlow System safely delivers therapeutic energies to the meibomian glands where obstructed meibum is liquefied and pushed up and out of the gland orifices
- Insulated, contoured design of the Activator vaults the cornea to protect the delicate structures of the eye
- Heat and pressure are regulated with redundant sensors
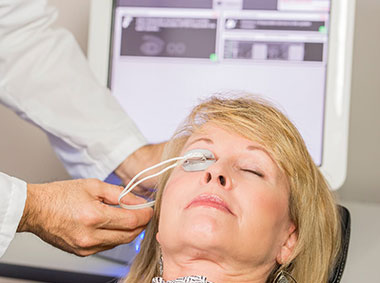
The LipiFlow® Thermal Pulsation System, a cleared medical device for Meibomian Gland Dysfunction (MGD), consists of a Console and a single-use sterile device, known as the Activator, and has a drug-free mechanism of action. Eye care professionals use the LipiFlow® System to treat MGD patients in-office with confidence and efficiency.
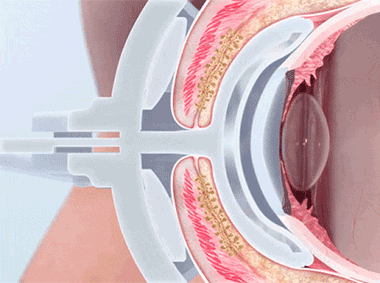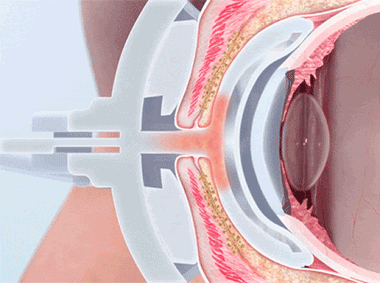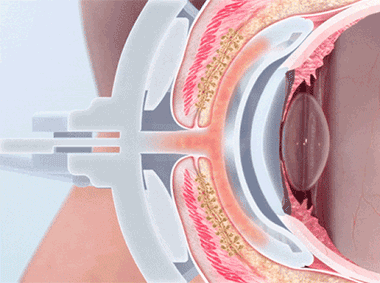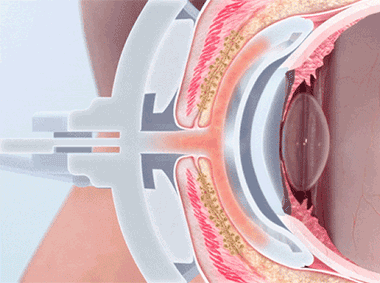
The LipiFlow® System represents more than 10 years of dedicated research and is protected by more than 30 patents. A phased pressure profile with adaptive force equalization and proximal-to-distal peristaltic motion evacuates gland contents as the inner lid is gently heated.
How the LipiFlow® System Works
- The procedure centers around the breakthrough Vector Thermal Pulse Technology™ (VTP)
- After an initial anesthetic drop, no drugs are required for the procedure
- The LipiFlow® system safely delivers therapeutic energies to the meibomian glands while protecting the delicate structures of the patient’s eye
- As a result, the obstructed meibum is liquefied and pushed up and out of the gland orifices
- Contoured design vaults the cornea and protects the eye
- Heat and pressure are regulated with redundant sensors
LipiFlow®’s Mechanism of Action
Key Messages for Patients
Did You Know?
- In one study, up to 86% of dry eye patients had signs of MGD1
- LipiFlow® has been shown to increase gland secretion threefold, on average, with just one treatment2
- Safety and long-term effectiveness have been demonstrated in peer-reviewed studies2,3,4
- LipiFlow® installations are evenly divided between ophthalmology and optometry clinics
- Capital and procedure disposable prices have been reduced by up to 50% to make the LipiFlow® system more accessible*
- LipiFlow® is available in more than 850 practice locations in the United States and Canada as of October 2017
*Pricing adjustments made in 2015 and 2016.
Benefits
The Science Behind LipiFlow®
VTP uniquely applies heat and peristaltic motion to the eyelid to remove gland obstructions and stagnant gland content.
![]()
A vaulted design protects the cornea while multi-point sensors monitor and regulate heat and pressure throughout the treatment. This maximizes results and minimizes discomfort. Along with force equalization, the eye is protected from heat and pressure while a nominal therapeutic temperature of 42.5 degrees Celsius is applied directly to the inner eyelid where the glands are located, while protecting the eyelid or delicate structures of the globe.
![]()
Proprietary heating technology ensures precise temperature regulation with continuous feedback and consistent heat application to the meibomian glands. The insulated and vaulted design of the Activator protects the cornea from unsafe temperatures.
![]()
Intelligent pressure feedback loop sends pulsed sequences to expel blockages and stagnant material from the gland. The custom pressure sequence maximizes gland clearing while device design protects the eye from unnecessary intraocular pressure.
The LipiFlow® Activator
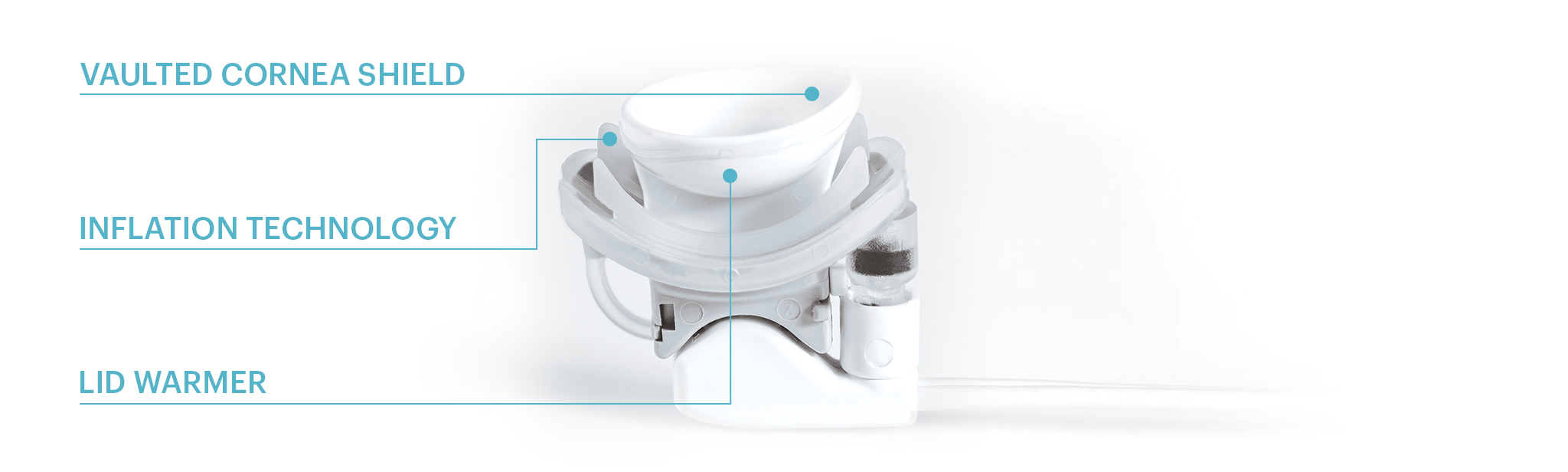
Vaulted Cornea Shield
Insulation protects the cornea from exceeding a safe 39.5 degrees Celsius, while an intelligent pressure feedback loop sends pulsed sequences to expel blockage and stagnant material from the gland. The vaulted design protects the delicate structures of the eye from both heat and pressure.
Inflation Technology
Pressure sensors in the console ensure the application of safe levels of pressure.
Lid Warmer
Multiple redundant sensors within the lid warmer protect against unsafe temperatures.
Indications & Safety Information
SUMMARY OF IMPORTANT SAFETY INFORMATION
Rx Only
FOR THE LIPIFLOW® THERMAL PULSATION SYSTEM
INDICATIONS FOR USE
The LipiFlow® System is intended for the application of localized heat and pressure therapy in adult patients with chronic cystic conditions of the eyelids, including Meibomian Gland Dysfunction (MGD), also known as Evaporative Dry Eye or Lipid Deficiency Dry Eye.
CONTRAINDICATIONS
Do not use the LipiFlow® System in patients with the following conditions. Use of the device in patients with these conditions may cause injury. Safety and effectiveness of the device have not been studied in patients with these conditions.
- Ocular surgery within prior 3 months, including intraocular, oculo-plastic, corneal or refractive surgery procedure
- Ocular injury within prior 3 months
- Ocular herpes of eye or eyelid within prior 3 months
- Active ocular infection (e.g., viral, bacterial, mycobacterial, protozoan, or fungal infection of the cornea, conjunctiva, lacrimal gland, lacrimal sac, or eyelids including a hordeolum or stye)
- Active ocular inflammation or history of chronic, recurrent ocular inflammation within prior 3 months (e.g., retinitis, macular inflammation, choroiditis, uveitis, iritis, scleritis, episcleritis, keratitis)
- Eyelid abnormalities that affect lid function (e.g., entropion, ectropion, tumor, edema, blepharospasm, lagophthalmos, severe trichiasis, severe ptosis)
- Ocular surface abnormality that may compromise corneal integrity (e.g., prior chemical burn, recurrent corneal erosion, corneal epithelial defect, Grade 3 corneal fluorescein staining, or map dot fingerprint dystrophy)
PRECAUTIONS
The Activator or Activator II (Disposable) may not fit all eyes, such as eyes with small palpebral fornices. Use of the LipiFlow® System in patients with the following conditions may result in reduced treatment effectiveness because these conditions may cause ocular symptoms unrelated to cystic meibomian glands and require other medical management. Safety and effectiveness of the device have not been studied in patients with these conditions.
- Moderate to severe (Grade 2-4) allergic, vernal or giant papillary conjunctivitis
- Severe (Grade 3 or 4) eyelid inflammation (e.g., blepharochalasis, staphylococcal blepharitis or seborrheic blepharitis). Patients with severe eyelid inflammation should be treated medically prior to device use
- Systemic disease conditions that cause dry eye (e.g., Stevens-Johnson syndrome, vitamin A deficiency, rheumatoid arthritis, Wegener’s granulomatosis, sarcoidosis, leukemia, Riley-Day syndrome, systemic lupus erythematosus, Sjögren's syndrome)
- Taking medications known to cause dryness (e.g., isotretinoin (Accutane®) and systemic antihistamines)
- Esthetic eyelid and eyelash procedures (e.g., blepharoplasty, lash extensions, eyelid tattooing)
In addition, the treatment procedure may loosen previously inserted punctal plugs, which may worsen the patient’s dry eye symptoms.
POTENTIAL ADVERSE EFFECTS
Potential adverse effects that may occur as a result of the procedure include, but are not limited to, the onset or increase in
- Eyelid/eye pain requiring discontinuation of the treatment procedure
- Eyelid irritation or inflammation (e.g., edema, bruising, blood blister, dermatitis, hordeolum or chalazion)
- Ocular surface irritation or inflammation (e.g., corneal abrasion, conjunctival edema or conjunctival injection (hyperemia)
- Ocular symptoms (e.g., burning, stinging, tearing, itching, discharge, redness, foreign body sensation, visual disturbance, sensitivity to light)
Potential serious adverse events (defined as permanent impairment or damage to a body structure or function or necessitates medical or surgical intervention to preclude permanent impairment or damage to a body structure or function) that are not anticipated because of the device mitigations to prevent occurrence include:
- Thermal injury to the eyelid or eye, including conjunctiva, cornea or lens
- Physical pressure-induced injury to the eyelid
- Ocular surface (corneal) infection



