What is an amniotic membrane?
The amniotic membrane (AM) is extracted from the innermost layer of human placental tissue for ophthalmological use. AM has gained its popularity from its use as a surgical graft to promote wound healing and ocular surface reconstruction. It is rich in neurotrophic factors such as nerve growth factor (NGF), various types of collagen, and basal epithelial cells that serve as a scaffold for limbal stem cells.1
AM possesses unique anti-inflammatory, anti-fibrotic, anti-angiogenic, and pro-healing properties for acute and chronic ocular surface disease.2 Indications for use include Dry Eye Disease (DED), neurotrophic keratitis, corneal neuropathic pain, persistent corneal epithelial defects, recurrent corneal erosions, filamentary keratitis, corneal ulceration, graft versus host disease, limbal stem cell deficiency, chemical injuries, post-corneal transplants, and glaucoma surgeries.1,3,4,6
Clinical application of amniotic membrane
There are two types of self-retained AMs available for the treatment of dry eye disease: dehydrated and cryopreserved. Both types require topical anesthesia prior to placement, are placed outside the slit lamp and will require a placement check behind the slit lamp.
Dehydrated membranes can be stored on a shelf at room temperature for up to 5 years, are relatively lower in cost compared to cryopreserved AM, and do not require any rinsing prior to preparation. Any excess tears should be soaked with a Weck-cel® or cotton tip applicator prior to inserting the membrane, otherwise, the membrane becomes too hydrated.
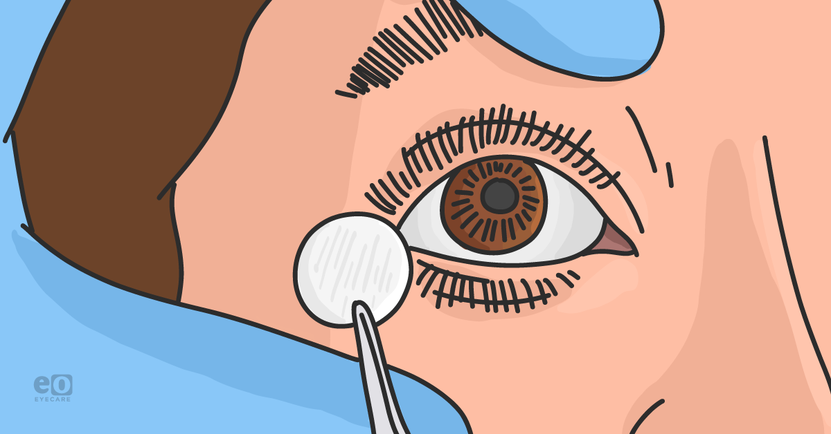
Dehydrated amniotic membrane insertion
Insertion typically requires non-toothed forceps to place the membrane directly on the cornea. Reclining the patient can help with gravity forcing the AM over the cornea as well as a lid speculum to control eyelid movements. The practitioner might use a Weck-cel® sponge or cotton tip applicator to smooth over any wrinkles.
Dehydrated AMs are available in various diameters and can be placed on the central cornea or on a local corneal defect to maximize its benefits. A soft bandage contact lens is placed over the dehydrated AM to secure it in place and prophylactic topical antibiotics are prescribed.
Any necessary concurrent eye drops can be continued, such as ocular hypotensives.
Manufacturers include: AmbioDisk (IOP Ophthalmics/Katena), BioDoptix, Triad Clarity, AcellFX (Akorn), and Aril Acellular Allograft Amniotic Membrane (Seed Biotech).
Cryopreserved AM must be stored at temperatures between -80 degrees C to 4 degrees C. They are preserved in an antibiotic and antifungal solution and therefore must be utilized with caution in allergic patients.
Cryopreserved amniotic membrane insertion
Cryopreserved AM requires rinsing with a saline solution prior to instillation. The carrier ring is placed underneath the superior and then inferior eyelid. The large 14 mm polycarbonate ring may be a source of discomfort for the patient, so a tape tarsorrhaphy can help alleviate some of this irritation.
Any concurrent topical medications can be continued throughout the course of treatment. Patients who are monocular and physically active are not good candidates due to the tape tarsorrhaphy.
Manufacturers include: Prokera/Slim (BioTissue)
Cryopreserved AMs are placed for 2-7 days, depending on the severity of the disease process. Results are characterized by resolution of acute corneal disease and can typically last up to 4 months after treatment.4 Very severe cases may require the sequential application of AM for complete resolution.
Setting patient expectations
Whether there is a large PMMA ring with the cryopreserved AM or a bandage SCL with the dehydrated AM, the patient can expect blurred vision. Advise patients not to touch or rub their eyes and if the tape over the eyelid needs to be replaced, they can replace it at home or come into the office.
Determining follow-up
Follow up will be determined by the underlying cause. For example, if there is a central corneal ulcer present, the patient should be seen daily with placement of AM. Any comorbid conditions (i.e., meibomian gland dysfunction) must be treated concurrently. For patients with multifactorial and chronic DED, AM is an effective treatment option for stabilizing the corneal epithelium but ongoing treatment will be necessary.
What does the literature say?
Corneal nerve density and corneal nerve sensitivity after placement of cryopreserved AM have been shown to improve, suggesting that corneal nerve regeneration is indicated with use of AM.5 This is likely due to the high content of nerve growth factor (NGF), a neurotrophin that is produced by the corneal epithelial cells and helps maintain epithelium and corneal nerves.5 Corneal neuropathic pain can also be successfully treated with AM to achieve sufficient pain control, which is supported by a post-treatment increase in corneal nerve density.6
The future of amniotic membranes in eyecare
I would urge practitioners to consider utilizing AM earlier in their treatment protocol than current third-tier recommendations outline. Given the data to support its role in corneal nerve regeneration, there are huge advantages to preventing further corneal disease and restoring corneal nerve and epithelial integrity with an early treatment approach.
References:
This content was originally published here.