Description
Description
The VeraPlug™ FlexFit™ punctal occluder is a medical device designed to occlude the punctum and
thereby provide reduction or elimination of tear drainage through the inferior or superior puncta, thus
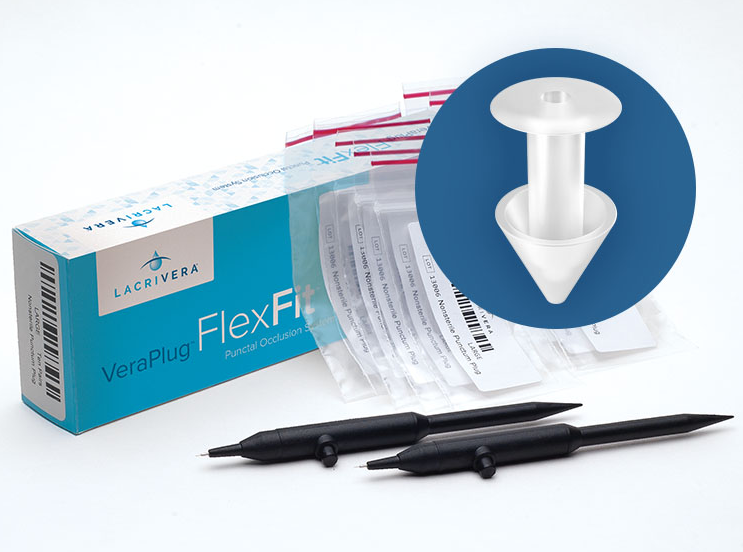
maintaining lubricating tears on the surface of the eye. Each VeraPlug™ FlexFit™ punctal occluder is molded from silicone. The VeraPlug™ FlexFit™ is available in four sizes (x-small, small, medium, and large).
Each box contains ten pairs of nonsterile occluders, two nonsterile inserters, identification labels for use by a qualified eye care physician, and instructions for use.
Indications for Use
The VeraPlug™ FlexFit™ is for use in patients with dry eye syndromes.
Contraindications
Contraindications include, but are not limited to, eye infections, sensitivity or allergies to the occluder material and/or materials used in the manufacture of the device, blockage/infection of the lacrimal systems, inflammation of the eyelid, and epiphora.
Precautions
The VeraPlug™ FlexFit™ may enhance the effect of ocular medications in the eye. Depending on the type of medication being used the dose may need to be altered accordingly. Conditions such as blepharitis or ocular surface inflammation should be treated prior to use of punctal plugs. If the patient experiences irritation, infection or epiphora after the insertion of the VeraPlug™ FlexFit,™ the occluder should be removed.
Potential Adverse Events
The following complications may occur and the patient must be informed prior to use:
■ Epiphora ■ Pyogenic granuloma ■ Washout
■ Foreign body sensation ■ Infection of the lacrimal system ■ Punctal erosion
■ Plug dislodgement or migration possibly requiring surgical intervention
Product Features & Documentation
Each box contains ten pairs of VeraPlug™ FlexFit™ punctal occluders for single use only. Additional accessories are not required for insertion, but stainless steel forceps may be used for insertion in place of an inserter. The use of stainless steel forceps may be recommended for removal. Instructions for use, patient implant cards, and patient labels are also included in each box. A patient label should be applied to an implant card and provided to every patient.
Proper Sizing
Proper sizing can be determined by using a 0.3mm gauge. If the gauge tip is snug in the
punctum, then a small size occluder is required.
If there is no resistance to the gauge then the next larger size should be tried. If resistance is
extremely tight, an x-small size occluder should be used.
Insertion
1 Anesthetize the area of the punctum with a topical anesthetic placed in the
conjunctival sac.
2 Apply a drop of saline solution or artificial tears onto the VeraPlug™ FlexFit™
to help ease insertion.
3 Position the insertion instrument by placing the forefinger on the release
button of the inserter and placing the occluder end of the insertion
instrument over the patient's (superior or inferior) punctum.
4 Vertically insert the VeraPlug™ FlexFit™ by positioning the occluder into the
punctum until the cap is flush with the punctal opening.
5 When the occluder is properly seated, depress the release button and
withdraw the insertion instrument.
6 Verify that the occluder is properly placed by confirming that the cap
is flush with the punctal opening. After insertion, monitor the
placement and integrity of the occluder to determine if/when the occluder
may need to be replaced.
Removal
Should removal be indicated, grasp the vertical shaft of the occluder
underneath the exposed cap with sterile forceps. Gently pull upward until the
plug is removed.
Storage
The device should be stored in a dry place at 15-30° Celsius.
Warnings
The VeraPlug™ FlexFit™ punctal occluder is intended for single use. Do not reuse.
If the device is reused this may increase the risk of complications including, but
not limited to, infection of the lacrimal system and plug dislodgement. Any serious
incident that has occurred should be reported to the manufacturer at info@lacrivera.
com or by calling the manufacturer in the US at +1-855-857-0518. Serious events
may also be reported to the competent authority in the country in which the user
and/or patient is established. A Summary of Safety and Clinical Performance may be
accessed by contacting the manufacturer. U.S. federal law restricts the sale of this
device by or on the order of a physician.